The purpose of this project was to design a hot air balloon and pressure relief valve. The requirements for the balloon were that it could rise to cruising altitude and travel for a one hour trip while carrying five passengers. Some assumptions we were given were that the balloon was spherical, it operates with propane gas, the weight for the propane is 60% of the weight of the fuel, it is made of rubber-silicon impregnated nylon and the air behaves like an ideal gas.
For the second half of the project we designed an adjustable pressure relief valve for steam that can operate between 30-40 atm. We determined the area of the orifice opening, the spring characteristics and have shown a sketch of the valve we designed.
For the second half of the project we designed an adjustable pressure relief valve for steam that can operate between 30-40 atm. We determined the area of the orifice opening, the spring characteristics and have shown a sketch of the valve we designed.
Hot Air Balloon

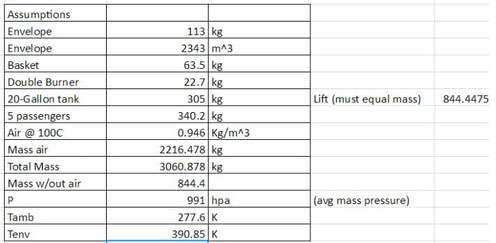
Assumptions:
Using Archimedes Principal it was determined that a volume of 2343 meters cubed would be required to displace the mass of the balloon and passengers.
Lift Equation:
In order to determine the lift required for the balloon to inflate and rise, some assumptions were made, including that the mass of warm air inside of the balloon prior to lift could be ignored and that all temperature, pressure, and cruising altitude related values were based off of the Boston location.
- F(net)=724 N
- Initial Mass of air can be neglected
- Initial filling of balloon required no use of propane aboard the balloon
- Heat Loss Coefficient = 10 W/m2K (provided from TA)
- Envelope Material = Non- Ripstop nylon
Using Archimedes Principal it was determined that a volume of 2343 meters cubed would be required to displace the mass of the balloon and passengers.
Lift Equation:
In order to determine the lift required for the balloon to inflate and rise, some assumptions were made, including that the mass of warm air inside of the balloon prior to lift could be ignored and that all temperature, pressure, and cruising altitude related values were based off of the Boston location.

Flat to Inflated Balloon:
It was assumed that the initial filling of the balloon was done by a truck and that all of the starter propane did not need to be calculated into the weight of fuel aboard the balloon. It was concluded that there was no heat loss to be calculated for this stage of the problem because all of the heated air was via propane from a truck. The amount that was lost and replaced when first achieving buoyancy did not matter. Variable temperatures that were initially provided above were current temperatures in Boston, Massachusetts, as this altitude was where the balloon took off from, and the temperature at the top of Mount Washington in New Hampshire, as its altitude was slightly higher than the altitude of the balloon, and proved to be the closest temperature we could find at a particular time of year at a particular altitude.
Balloon Rising:
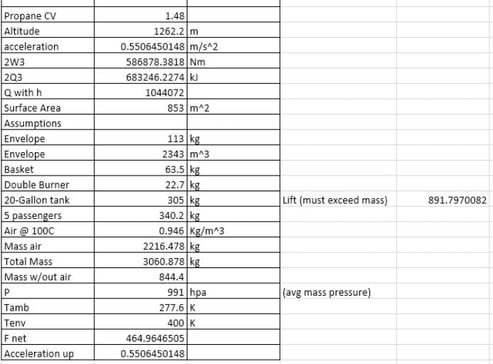
In order to calculate the amount of heat lost traveling to the altitude of 4,141 feet, or 4,000 feet above Boston, the acceleration to rise such a distance was found. To do so, a force balance was made. The balloon had previously been in equilibrium, so, to make the balloon rise, the temperature inside of the balloon increased. By doing so, there was a net upward force (465 N). Knowing the force up and applying basic physics equations, it was determined that it would take approximately 92 seconds for the balloon to reach the desired height. Knowing this period of time, the equation Q = h*AS*T+ W was used. The first part of the equation accounts for the heat loss using a heat loss coefficient. With the units found for this, Q can be found by multiplying by a period of time, dividing by 1000 to account for the J to kJ conversion, and adding work. Work is considered here because the balloon is moving upward, thus a force is acting over a distance.
It was assumed that the initial filling of the balloon was done by a truck and that all of the starter propane did not need to be calculated into the weight of fuel aboard the balloon. It was concluded that there was no heat loss to be calculated for this stage of the problem because all of the heated air was via propane from a truck. The amount that was lost and replaced when first achieving buoyancy did not matter. Variable temperatures that were initially provided above were current temperatures in Boston, Massachusetts, as this altitude was where the balloon took off from, and the temperature at the top of Mount Washington in New Hampshire, as its altitude was slightly higher than the altitude of the balloon, and proved to be the closest temperature we could find at a particular time of year at a particular altitude.
Balloon Rising:
In order to calculate the amount of heat lost traveling to the altitude of 4,141 feet, or 4,000 feet above Boston, the acceleration to rise such a distance was found. To do so, a force balance was made. The balloon had previously been in equilibrium, so, to make the balloon rise, the temperature inside of the balloon increased. By doing so, there was a net upward force (465 N). Knowing the force up and applying basic physics equations, it was determined that it would take approximately 92 seconds for the balloon to reach the desired height. Knowing this period of time, the equation Q = h*AS*T+ W was used. The first part of the equation accounts for the heat loss using a heat loss coefficient. With the units found for this, Q can be found by multiplying by a period of time, dividing by 1000 to account for the J to kJ conversion, and adding work. Work is considered here because the balloon is moving upward, thus a force is acting over a distance.

Cruising at 4,141 ft:
To cruise over a period of time, it is expected that the altitude of the balloon will not change. Similar to achieving buoyancy on the ground, there is now a buoyant force at the new altitude. Having inputted values in excel to calculate lift, different temperatures were tried for the new envelope temperature so that the lift would match mass. At this point, the temperature decreased to correct for the upward acceleration. The pressure at this altitude has also changed, and the average pressure in Massachusetts at 4,000 feet was used.
Heat loss was calculated using the same equations as during lift. However, in this calculation, work was not included as net force was zero. Not including the minute and a half it took to rise to the proper altitude for cruising, heat loss was calculated for a one hour interval.
Propane Ingested:
To calculate the total amount of propane used, all of the “Q values” were added together in kJ. Using engineering toolbox, the heat of combustion of propane (50340 kJ/kg) was used to find a mass of propane. Having a mass, volume could be found using the density of propane. Overall, 10.7 gallons of propane were used. In total, it was decided that there would be 100 gallons of propane stored in the balloon as a precautionary extra amount. The mass of propane had to be divided by a value of 1,000 as it was discovered that somewhere in the changing of variables the output of propane was off by this factor.
To cruise over a period of time, it is expected that the altitude of the balloon will not change. Similar to achieving buoyancy on the ground, there is now a buoyant force at the new altitude. Having inputted values in excel to calculate lift, different temperatures were tried for the new envelope temperature so that the lift would match mass. At this point, the temperature decreased to correct for the upward acceleration. The pressure at this altitude has also changed, and the average pressure in Massachusetts at 4,000 feet was used.
Heat loss was calculated using the same equations as during lift. However, in this calculation, work was not included as net force was zero. Not including the minute and a half it took to rise to the proper altitude for cruising, heat loss was calculated for a one hour interval.
Propane Ingested:
To calculate the total amount of propane used, all of the “Q values” were added together in kJ. Using engineering toolbox, the heat of combustion of propane (50340 kJ/kg) was used to find a mass of propane. Having a mass, volume could be found using the density of propane. Overall, 10.7 gallons of propane were used. In total, it was decided that there would be 100 gallons of propane stored in the balloon as a precautionary extra amount. The mass of propane had to be divided by a value of 1,000 as it was discovered that somewhere in the changing of variables the output of propane was off by this factor.
Pressure Relief Valve
Assumptions:
We started by finding the forces produced by the pressures given in the problem: 30 and 40 atm using the equation F=PA.
Next, we calculated the spring constant “k”, by using the force produced at 40 atm:
F=k*x
31.4=k*2.5
k=12.56
Then, we calculated the distance between the piston and the opening at 30 atm using the spring constant
F=k*x
23.55=12.56*x
x= 1.875 in
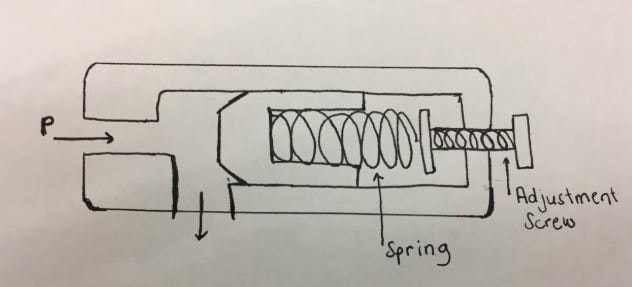
With this information we knew that we must adjust the screw and move the piston 0.625 inches closer than the 2.5 inches we assumed to start. We decided that we would use oil tempered chrome silicon as the spring material. We picked this material because of its high elastic modulus of 30 and its high operating temperature of 475 F. During calculations it was assumed that the valve was initially closed and the distance required for the valve to open was 2.5 inches. Having found the spring constant, the distance the spring would move at a lower pressure was found (1.875 in). Knowing this distance, the pressure relief valve can work at variable pressures by using the screw seen in the diagram below to move the initial placement of the piston so that it moves the necessary distance to open for different pressures. By moving the piston to a further distance, the valve will not fully open when a pressure of only 30 atm is used.
- Assume diameter of opening is 1 in
- Area of opening is 0.785 in2
- Distance from opening to the piston is 2.5in when the pressure is 40 atm
We started by finding the forces produced by the pressures given in the problem: 30 and 40 atm using the equation F=PA.
Next, we calculated the spring constant “k”, by using the force produced at 40 atm:
F=k*x
31.4=k*2.5
k=12.56
Then, we calculated the distance between the piston and the opening at 30 atm using the spring constant
F=k*x
23.55=12.56*x
x= 1.875 in
With this information we knew that we must adjust the screw and move the piston 0.625 inches closer than the 2.5 inches we assumed to start. We decided that we would use oil tempered chrome silicon as the spring material. We picked this material because of its high elastic modulus of 30 and its high operating temperature of 475 F. During calculations it was assumed that the valve was initially closed and the distance required for the valve to open was 2.5 inches. Having found the spring constant, the distance the spring would move at a lower pressure was found (1.875 in). Knowing this distance, the pressure relief valve can work at variable pressures by using the screw seen in the diagram below to move the initial placement of the piston so that it moves the necessary distance to open for different pressures. By moving the piston to a further distance, the valve will not fully open when a pressure of only 30 atm is used.